Which of the Following Best Describes a Double-replacement Reaction
Which of the statements below best describes the following reaction. The reactants are two compounds and the products are two different compounds.

Combustion Reactions Chemical Reactions Chemical Equation Reactions
Tap again to see term.

. DESCRIPTION- the reaction is double displacement reactiondouble replacement reactionmetathesis double View the full answer Transcribed image text. In a double displacement reaction atoms from two different compounds switch places. What would you expect to form when a metal reacts with a nonmetal.
Tap card to see definition. According to rule 8 in Table 72 Ag3PO4 is insoluble meeting the requirements for a double-replacement reaction to happen. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.
Double Displacement reaction or Double Replacement reaction. Ask us anythingWhich of the following demonstrates a double-replacement reaction. EqC_10H_8 12 O_2 rightarrow 10 CO_2 4 H_2O eq A.
Which of the following best represents a double replacement reaction. Water and sodium bromide. Group of answer choices double replacement reaction acid base reaction neutralization redox reaction there is no reaction.
NaOH aq HCl aq NaCl aq. Ice with a mass of 15 grams is melted AND then the temperature of the now liquid water is raised from 0 degrees Celsius up to 95 degree Celsius. Choose the general equation for the double replacement reaction.
In the formation of sodium chloride by the combination of sodium and chlorine. One compound splits up forming two or more elements or new compounds. We have a new and improved read on this topic.
2BiCl₃ 3CaI₂ 2BiI₃. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. Which of the following best describes a double-replacement reaction.
Which of the following best describes the reaction if any that occurs when aqueous solutions of ironIII nitrate and sodium iodide are combined. Click here to view We have moved all content for this concept to for better organization. Please update your bookmarks accordingly.
It is a double replacement because the reaction starts with two compounds and ends with two compounds where the positive and negative ions have changed places. HINT you will need to perform two calculations and then add up the results. Click card to see definition.
Helpful 0 Not Helpful 1 Add a Comment. Two elements join together to form a new compound. Double displacement reactions can be further classified as.
A single substance breaks down into more than one substance Which of the following correctly. See the answer See the answer done loading. How much heat is needed for this total process.
An example of a double displacement reaction is the reaction between sodium hydroxide and hydrochloric acid to for sodium chloride as shown. The positive hydrogen ion on the Chlorine has been replaced by a positive sodium ion on the Chlorine. What term refers to a chemical reaction that absorbs heat energy.
Click again to see term. What are the products of the double-replacement reaction between aqueous hydrogen bromide and aqueous sodium hydroxide. Describes the double-replacement reaction and gives examples.
A lone element takes the place of a different element in a compound. Click here to get an answer to your question which of the following best describes a double-replacement reaction. HC2H3O2aq NaOHaq NaC2H3O2aq H2Ol Aqueous solutions of acetic acid and sodium hydroxide react to produce aqueous sodium acetate and water.
Therefore your answer is photosynthesis is a double replacement reaction. Click Create Assignment to assign this modality to your LMS. 2H 2 g O 2 g 2H 2 O l heat a.
Photosynthesis is not a single reaction pathway but two one dependent on the other. A double replacement reaction is represented by the general equation. Fe2O3 6HCl 2FeCl3 3H2O.
Predict the type of reaction between LiBr2 aq 2NaOH aq. Double replacement reaction O acid base reaction. While the Sodium ion on the hydroxide has been replaced by the Hydrogen ion.
A single replacement reaction occur when an element in a substance is replaced by other element. The answer is Na3Po4MnCl-Mn3Po4NaCl. Sodium loses electrons but chlorine gains electrons.
Atoms in one compound switch places with atoms in another compound. Which of the following best describes a double-replacement reaction. Therefore a chemical reaction will occur and silver phosphate and lithium nitrate are the products.
AB A B A B AB A BC AC B AC BD AD BC. Choose the term that best describes the following chemical reaction. No it is a double replacement reaction.
When the two reactants are mixed the potential products are Ag3PO4 and LiNO3. Atoms in one compound switch places with atoms in another compound. Occurs when the positive ion or known as cations or the negative ions or anions of different compound in the reactant switch places forming two entirely different compound.
Which of the following best describes the reaction if any that occurs when aqueous solutions of iron III nitrate and sodium iodide are combined. AB CD AD CB.
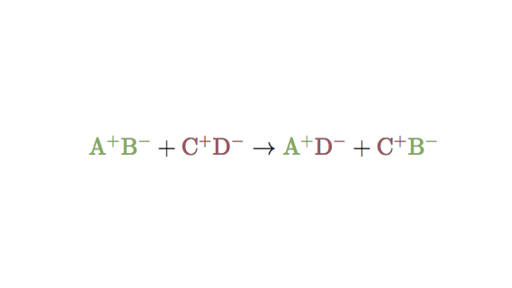
Double Replacement Reactions Double Displacement Article Khan Academy

Double Replacement Reaction Is A Type Of Chemical Reaction Where Two Compounds React And The Positive Negative I Positive And Negative Positivity Negative Ions

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube
Comments
Post a Comment