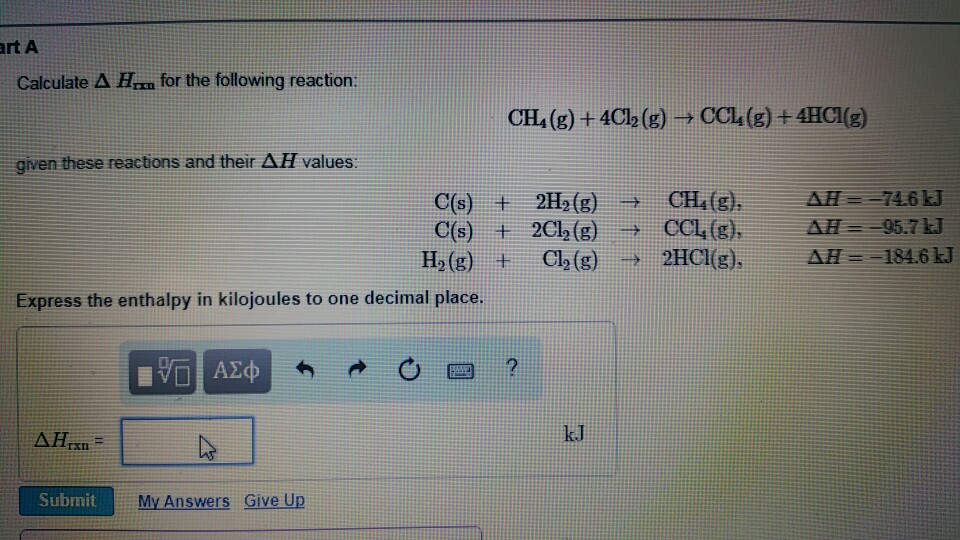
Calculate Delta H Rxn for the Following Reaction
Delta E H will essentially be equal when there is no change in volume. SiH_4 g 2O_2 g rightarrow SiO_2 s 2H_2O l Use the following information to determine Delta H_f0 for PbO s yellow.

Calculate The Dh Rxn For The Following Reaction Youtube
Likewise people ask how do you find Delta H RXN.

. 1 Cs2H2g--CH4g delta H -746 kJ 2 Cs2Cl2g--CCl4g delta H -957 kJ 3 Chemistry. For the reaction described by the chemical equation. 2 HCNg AgCNs - Ags H2 g 3 CNsΔ H -113 kJmol.
Use the Δ H and balanced chemical equation below and calculate the Δ Hf of CNs. For the reaction described by the chemical equation. Calculate the delta H rxn for the following reaction.
Up to 25 cash back Use the standard reaction enthalpies given below to determine the ΔHrxn for the following reaction4NOg 2O2g 4NO2g ΔHrxn. Calculate the ΔH of the following reaction. Delta H rxn -6331 kJmol a Calculate the value of Delta S rxn at 250 C.
At first I selected D The ΔHf of O2 g is needed for the calculation. Delta H Chemistry. The following equation can be used to calculate the standard enthalpy of reaction.
Using the equation delta H delta E PV. The enthalpy of reaction is calculated under standard conditions STP. Here are a number of highest rated Delta H Chemistry pictures upon internet.
KJGivenN2g O2g 2NOg ΔHrxn 183 kJ½N2g O2g. Use the formula H m x s x T to solve. 3C2H2g - C6H6l.
O K to 299 o K. -24183 KJmol and H 2 CO 3 g. 3C2H2g - C6H6l.
Note that the delta h in a chemical reaction is entirely independent of how. We acknowledge this nice of Delta H Chemistry graphic could possibly be the most trending subject following we share it in google pro or facebook. 1 Cs2H2g--CH4g delta H -746 kJ 2 Cs2Cl2g--CCl4g delta H -957 kJ 3 Chemistry.
Here are a number of highest rated H2o Delta H pictures on internet. H3AsO4 aq 4H2 g rightarrow AsH3 g 4H2O l Answer. Delta H degree f H3AsO4 aq - 9046 kJmol.
This exchange may be either absorption of thermal energy from the atmosphere or emission of thermal energy into the atmosphere. -393509 KJ mol H 2 O g. CH4g4Cl2g--CCl4g4HCl Use the following reactions and given delta Hs.
C2H6G O2G----CO2GH2OG Delta H F. Calculate Delta H_rmrxn for the following reaction. C In which direction is the reaction as.
Its submitted by dealing out in the best field. Calculate Delta H_rxn0 for the following reaction at 298 K. H2o Delta H.
Simply plug your values into the formula H m x s x T and multiply to solve. Calculate Delta H rxn for the following reaction after it is properly balanced with smallest whole number coefficient. Up to 256 cash back Calculate the Delta H degree rxn for the following reaction.
B Calculate Delta G rxn. How do you calculate delta H for a reaction Use the formula H m x s x T to solve. Calculate the delta H rxn for the following reaction.
Use the formula H m x s x T to solve. RmCH_4g 2rmO_2g rightarrow rmCO_2g 2rmH_2Ol Use the. For the reaction described by the chemical equation.
However apparently the answer is -1172 but I have no idea why. If the calculated value of ΔH is positive does that correspond to an endothermic reaction or an exothermic reaction. Δ Hf of CNs 101 kJmol do not forget to divide by the 3 coefficient Posted in Chemistry.
ΔH rxn 4x90 6x-286 - 4x-46 X ΔH rxn -1356 - -184X ΔH rxn -1356 184 - X. Calculate Delta H_ rm rxn for the following reaction. Rm CH_4 g rm O_2 g rightarrow rm CH_2O g rm H_2O g Delta H -284 rm kJ.
Calculate the delta H rxn for the following reaction. Once you have m the mass of your reactants s the specific heat of your product and T the temperature change from your reaction you are prepared to. 1 Cs2H2g--CH4g delta H -746 kJ 2 Cs2Cl2g--CCl4g delta H -957 kJ 3 Chemistry.
Delta H degree f AsH3 g 664 kJmol. We identified it from well-behaved source. Its submitted by management in the best field.
Once you have m the mass of your reactants s the specific heat of your product and T the temperature change from your reaction you are prepared to find the enthalpy of reaction. CH4g4Cl2g--CCl4g4HCl Use the following reactions and given delta Hs. Delta H degree f H2O l - 2858 kJmol.
We admit this kind of H2o Delta H graphic could possibly be the most trending topic in imitation of we part it in google help or facebook. For CO_2 g -3935 kJmol and Delta H_f0 for CO g -1105 kJmol. For the following reaction equations calculate the energy change of the reaction at 25.
Once you have m the mass of your reactants s the specific heat of your product and T the temperature change from your reaction you are prepared to find the enthalpy of reaction. ΔH rxn ΔH f productsΔH f reactants Δ H r x n Δ H f products Δ H f reactants. How can -1172 be the answer here.
This change of thermal energy in the thermodynamic system is known as change of enthalpy or delta h written as H in chemistry and calculated using the formula H cmT. June 8 2021 by Admin. CH4g4Cl2g--CCl4g4HCl Use the following reactions and given delta Hs.
3C2H2g - C6H6l. Rm CH_4 g 2 rm O_2 g rightarrow rm CO_2 g 2 rm H_2O l Use the following reactions and given Delta H rm s. CO 2 g H 2 O g -- H 2 CO 3 g if the standard values of ΔH f are as follows.
We identified it from reliable source. ΔH rxn -1172 X. PbO s yellow CO g rightarrow Pb s CO_2 g Delta H_rxn0 -6569 kJ Delta YY.
The standard internal energy change for a reaction can be symbolized as ΔUrxn or ΔErxn.

Solved Using Hess S Law Calculate The Heat Of Raction Chegg Com
Solved Calculate Delta H Rxn For The Following Reaction Ch4 Chegg Com
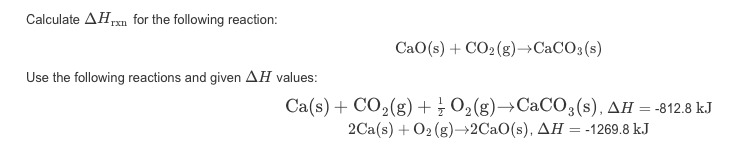
Solved Calculate Delta H Rxn For The Following Reaction Use Chegg Com
Comments
Post a Comment